For decades, ketamine has been known mainly as an anesthetic and a notorious party drug dubbed “Special K.” But in recent years, its unexpected ability to rapidly alleviate depression has transformed it from a “devil” to an “angel” in mental health treatment. This remarkable shift in perception hinges significantly on our deep understanding of how ketamine combats depression at its core.
Professor HU Hailan and her colleagues at Zhejiang University have discovered that, when ketamine is administered, it specifically targets a brain region known as the lateral habenula (LHb), often referred to as the “anti-reward center”. Their research reveals that ketamine’s initial action site occurs at NMDA receptors on neurons within this region, shedding light on the brain’s precise response to the drug and mapping out the neural pathways from the LHb to the hippocampus.
In previous studies published in Nature in 2018 and 2023, HU’s team detailed the rapid and long-term antidepressant effects of ketamine. Their latest research, published in Science on August 9, 2024, titled “Brain region–specific action of ketamine as a rapid antidepressant”, adds a crucial piece to the ketamine antidepressant “puzzle.” Together, these studies construct a theoretical framework centered on the LHb NMDA receptors, providing valuable guidance for both the clinical use of ketamine and paving the way for developing new antidepressant drugs.
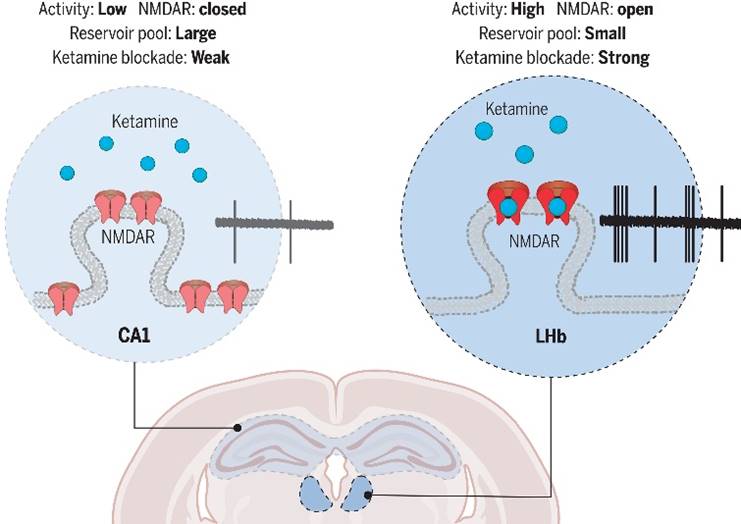
Brain region–specific action of ketamine.
While it’s known that ketamine exerts its effects primarily by binding to NMDA receptors, which are broadly expressed and distributed throughout the brain, one key question remains: Does ketamine act synchronously across the brain or does it target specific regions first? The scientific community has yet to reach a consensus, but answering this question is crucial for understanding ketamine’s action mechanism.
Is it parallel or sequential?
The answer to this question isn’t straightforward. In 2018, HU’s team discovered that ketamine targets the LHb, where it suppresses the burst firing of neurons to alleviate depression. Meanwhile, other research teams suggested that ketamine affects the hippocampus and the cortex, inducing positive changes in neural plasticity. But whether these effects are sequential or simultaneous lacked direct experimental evidence.
“The rapid action of ketamine provides a crucial temporal advantage,” explains Dr. CHEN Min, the paper's lead author. In an experiment, the team injected ketamine into the abdomen of depressive-like mice and monitored immediate brain changes. “Within a few minutes to an hour or two, neuronal activity in the LHb drops significantly. Surprisingly, there is virtually no change in the hippocampus and the cortex during this period.”
Through both in vitro slice electrophysiology and in vivo tetrode recording, the team finds that the LHb is the first brain region to respond. “This indicates that ketamine’s effects on NMDA receptors are region-specific, rather than occurring simultaneously across multiple brain regions. The first brain region to respond signifies a more direct interaction with ketamine,” says HU.
How does ketamine anchor to a specific brain region?
As an organic small molecule, how does ketamine “choose” to act on a specific brain region? According to HU, it depends largely on how ketamine interacts with neurons and the activity level of those neurons in different regions of the brain. To delve into this, the research team compared the LHb and the hippocampus in depressive-like mice.
“NMDA receptors are ion channels that open when neurons are active,” explains Dr. MA Shuangshuang, the study’s second author. The target site of ketamine is located inside these ion channel, meaning that ketamine can only enter and exert its effects when the NMDA receptors are open during neuronal activity. This makes ketamine’s blocking action highly activity-dependent. The team’s experiments showed that neuronal activity in the LHb of the brain is significantly higher than in hippocampal pyramidal neurons, giving ketamine more opportunities to bind and block NMDA receptors in the LHb. By modifying the neuronal activity in both brain regions, the researchers successfully reversed their sensitivity to ketamine.
Further analysis of the neurons’ synapses in these two regions uncovered another key difference: the synaptic reserve of NMDA receptors is much lower in LHb neurons compared to those in hippocampal neurons. This suggests that even a small amount of ketamine can effectively “cover” NMDA receptors in the LHb, leading to higher blocking efficiency.
Therefore, HU’s team identified several neurological factors that contribute to ketamine’s brain region specificity: the activity-dependent nature of ketamine’s action, the activity levels of neurons, and the synaptic reserve of NMDA receptors in local regions.
Upstream and downstream interactions
When it comes to unraveling the molecular targets of ketamine, HU’s team zeroed in on NMDA receptors in specific brain regions, identifying those in the lateral habenula (LHb) as the key players in ketamine’s antidepressant effects. But to truly understand how ketamine works, it’s crucial to map out the entire neural circuitry — especially the upstream and downstream interactions.
Existing research suggests that, in addition to the LHb, other brain regions also contribute to ketamine’s antidepressant effects. For example, intraperitoneal injections of ketamine have been shown to increase serotonin and brain-derived neurotrophic factor (BDNF) levels in the hippocampus. Yet, there is no conclusive evidence pointing to the primary initiating factor. To address this, HU’s team designed an experiment where they specifically knocked out the NR1 subunit (an NMDA receptor subunit) in the LHb neurons of mice. They found that ketamine’s rapid antidepressant behavioral effects disappeared, and there was no significant increase in serotonin and BDNF in the hippocampus following intraperitoneal ketamine injections.
“This indicates that the LHb is the starting point for ketamine’s action, and the hippocampus’s response likely plays a downstream role in its antidepressant effects,” says HU. To put it simply, if ketamine’s pathway in the brain is likened to a game of bowling, the NMDA receptors on LHb neurons act as the “lead pin.” When ketamine hits this “pin”, it sets off a series of reactions, knocking down other “pins.”
From theory to clinical practice
Ketamine provides humanity with a key to cracking the code of depression. Since the discovery of ketamine’s rapid antidepressant effect in the early 21st century, numerous studies have sought to understand how it works. Among these endeavors, HU’s team has distinguished itself by developing a unique theoretical framework centered on the role of NMDA receptors in the LHb in ketamine’s antidepressant action.
In 2018, HU’s team made a breakthrough discovery with a Nature publication that revealed how ketamine’s rapid antidepressant effects are linked to burst firing in the LHb. Their research demonstrated, for the first time, that ketamine alleviates depression by inhibiting this burst firing through its interaction with NMDA receptors in the neurons of the LHb. In 2023, the team published another Nature paper that delved into ketamine’s long-term antidepressant mechanism, uncovering a unique “embedding” action mechanism that prolongs its efficacy beyond its half-life. Their most recent contribution, featured in Science, explores the brain-region-specific mechanisms and the intricate upstream-downstream relationship of drug action, adding an important “piece” to the “puzzle” of ketamine’s antidepressant mechanism. This work provides valuable theoretical guidance for clinical applications and the development of new antidepressant therapies.
Regarding the mechanisms of ketamine’s antidepressant action, there are two main hypotheses in academia. The first is the ‘disinhibition’ hypothesis, which posits thatketamine lifts the “clouds” of depression by inhibiting overactive “brakes” in the brain. The second is the ‘neuroplasticity’ hypothesis, which proposes thatketamine enhances the brain's capacity to produce substances and form connections that promote well-being, thereby fostering neural growth and synaptic formation. The latest research by HU’s team provides compelling experimental evidence supporting the key role of the LHb in the ‘disinhibition’ process, while also acknowledging the impact of neuroplasticity. The study further clarifies the sequential relationship between neuroplastic changes in other brain regions downstream of the LHb along the antidepressant pathway. “This work bridges these two hypotheses, offering a more unified explanation for the various mechanisms discovered in previous ketamine research,” HU remarks.
Studies by HU’s team have also captured immense attention in the clinical domain. Previously, deep brain stimulation (DBS), a common clinical treatment for depression, has rarely focused on the habenula region. However, since the team’s 2018 findings highlighted the critical role of the LHb, larger sample studies on DBS treatment for depression targeting the habenula have been conducted. At Ruijin Hospital in Shanghai and the 301 Hospital in Beijing, DBS has been used to inhibit habenula activity in the treatment of 13 cases of treatment-resistant depression, with significant efficacy observed in 11 of the cases. “The clinical feedback further validates our LHb-centered theory of depression and inspire us to continue our pursuit of understanding the core mechanisms that could eventually lead to a cure for depression,” says MA.
(From ZJU NEWSROOM)

