With the arrival of summer, cleaning air conditioners is an imperative step, as they often harbor various pathogens, including Legionella pneumophila. A recent scientific breakthrough has shed light on a novel bacterial “toxin” present within Legionella pneumophila — an effector protein named LnaB. This protein serves a dual function. It can actively cleanse harmful molecules so as to aid host cells in maintaining their normal physiological process. Meanwhile, it can impair the host immune response to bacterial invasion, thereby preventing Legionella pneumophila from being eliminated by the host.
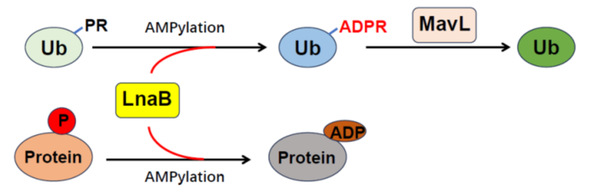
Fig. 1: Schematic diagram of the LnaB mechanism
On July 11, the collaborative research endeavors of Prof. ZHU Yongqun from the College of Animal Sciences at Zhejiang University, Dr. ZHOU Yan from the College of Life Sciences at Zhejiang University, and Prof. LIU Xiaoyun from the School of Basic Medical Sciences at Peking University made headlines with their publication in Nature. Their paper, titled “Legionella effector LnaB is a phosphoryl-AMPylase that impairs phosphosignalling”, unveils a groundbreaking discovery. “It’s quite uncommon for a bacterium to use the same effector protein to fulfill two different roles,” ZHU Yongqun remarked. He referred to this phenomenon as a ‘hidden needle in cotton’ strategy — a clever tactic that grants the bacterium a transient symbiosis with the host cell, thereby enhancing its likelihood of survival and proliferation.
LnaB — A mysterious “weapon”
A key term in this study is “post-translational modification,” a term well-recognized in the domain of biology. This process typically involves covalently adding a chemical group to an amino acid in a protein after the protein has been synthesized. To date, researchers have identified hundreds of post-translational modification techniques, including ubiquitination, acetylation, methylation, etc. If proteins are the functional “machineries” within cells, it is often their post-translational modifications that dictate their operational efficacy. ZHU Yongqun’s research team has long been committed to investigating the interactive mechanisms between pathogens and their hosts to explore novel post-translational modifications. This exploration serves as their gateway to discovering the “secret weapon” of bacterial pathogens.
Legionella pneumophila, the bacterium that causes Legionnaires’ disease in humans, adopts a unique survival strategy by infecting and entering alveolar macrophages, where it creates specialized vacuolar structures to facilitate its survival and proliferation. In 2016, several laboratories worldwide discovered that Legionella pneumophila promotes the maturation of Legionella-containing vacuoles through a non-canonical ubiquitination process. “A byproduct of this process is phosphoribosylated ubiquitin,” said ZHU Yongqun. He further noted, “Our discovery in 2017 revealed that this byproduct is toxic to human cells. However, Legionella pneumophila manages to invade and proliferate in large numbers in human cells.” The research team speculated that Legionella pneumophila might have a special effector protein that removes this toxic ubiquitin molecule or modifies ubiquitin in other ways. This modification potentially allows for a temporary co-existence with the host cell, thus ensuring the survival and reproduction of the bacterium.
Through a series of in vitro and in vivo screening systems, the research team finally focused on the effector protein LnaB. It plays a leading role in this study, opening up a new vista for scientists to understand bacterial pathogenic mechanisms.
LnaB: Detoxifying and poisoning host Cells
The research team has made a remarkable discovery that LnaB can “detoxify” host cells via a very special modification process. According to ZHU Yongqun, LnaB is an exceptional adenosine monophosphate cyclase (AMPylase) that performs adenylylation on the phosphoryl group of the phosphoribosylated ubiquitin, generating ADP-ribosylated ubiquitin, which is then hydrolyzed into non-toxic, native ubiquitin by the effector protein MavL. This cascade reaction catalyzed by LnaB and MavL reverses the noncanonical ubiquitination process of Legionella pneumophila, therefore safeguarding the canonical ubiquitination pathway that is essential for numerous physiological processes of the host cells. “Our discovery that adenylylation on the phosphoryl group vastly expands the conventional understanding about adenylylation that usually occurs on the side chain of residues in proteins,” said ZHU Yongqun.
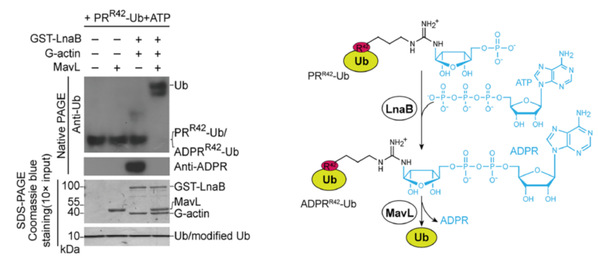
Fig. 2: The cascade reaction catalyzed by LnaB and MavL reverses the non-canonical ubiquitination process.
Moreover, LnaB can also suppress the host cell’s immune response. ZHU Yongqun said that during the infection, LnaB modifies essential the Src family kinases within the host cell, producing a unique “ADPylation modification,” which challenges the old concept that the phosphate group on proteins is resistant to further posttranslational modifications within the cell. The Src family kinases, which are critical for signal transduction and possess significant biological roles, are inactivated by LnaB. This inactivation impairs the downstream signal transduction, and silences the host’s immune response. “LnaB is also the first virulence factor identified that can directly modify the phosphorylated tyrosine residues on the activation loop of the Src family kinases,” ZHOU Yan added.
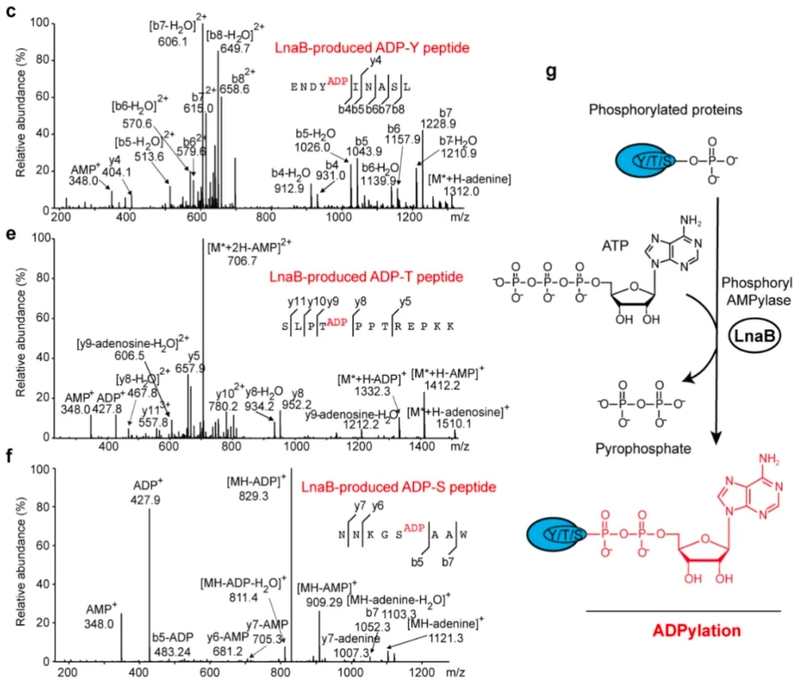
Fig. 3: LnaB exhibits robust phosphoryl-AMPylase activity toward phosphorylated residues and
produces unique ADPylation modification in proteins
The research team was captivated by the dual function of a single effector protein —simultaneous “detoxification” and “poisoning,” revealing a complex mechanism of the action. Through homologue search, they uncovered 162 LnaB homologues in a large number of bacterial pathogens of more than 20 species, suggesting at the prevalence of the phosphoryl-AMPylation in pathogen-host interactions.

Fig. 4: LnaB represents a large family of AMPylases adopting a common structural fold, which is distinct from those of the previously known AMPylases.
This study significantly deepens our understanding of bacterial pathogenesis. Given the critical roles Src family kinases in numerous human diseases, the LnaB’s unique activity possibly could be developed to a potential therapeutic method for the related diseases. Moreover, the large number of the LnaB family members also provides chances for future exploration of bacterial pathogenesis.
(From ZJU NEWSROOM)

