Legend has it that Nvwa, the mother goddess in Chinese mythology, created mankind. The infinite possibilities of nature are often captured in mythological stories. With the development of modern science, scientists have discovered that the proteins essential for life are basically composed of 20 natural amino acids.
However, the universe of amino acids extends far beyond these 20. In recent years, through various research methods, scientists have synthesized a variety of non-canonical amino acids. This breakthrough has sparked curiosity about whether organisms could be engineered to autonomously encode these non-canonical amino acids, thus opening up more possibilities for the design and function of biological proteins.
The challenges of this research are truly mind-boggling! On June 7, a team led by Dr. LIN Shixian, Principal Investigator from the Life Sciences Research Institute at Zhejiang University, published an article in the journal Science. They explored a novel way for organisms to autonomously encode non-canonical amino acids. This groundbreaking work lays the foundation for the potential creation of artificial life forms capable of encoding non-canonical amino acids in the lab.
Finding the “Achilles’ Heel” in the blind spot
Previous research has shown that natural organisms use a translation system to assemble 20 natural amino acids into proteins based on genetic coding information. This system recognizes 64 universal triplet genetic “codons” that drive the evolution of complex life forms.
Since the discovery of these translation rules, scientists have been exploring the possibility of rewriting the genetic code table to allow organisms to incorporate non-canonical amino acids beyond the standard 20.
Out of the 64 original codons, 61 sense codons synthesize amino acids, while 3 blank codons terminate the synthesis process. The 61 sense codons have traditionally been seen as unsuitable for encoding non-canonical amino acids. Therefore, research has often focused on the 3 blank codons, but these efforts have frequently hit significant roadblocks.
Some scientists have attempted to adopt new blank codons, such as quadruplet codons and non-natural bases, to expand the genetic code. However, these approaches have faced greater challenges in terms of efficiency, general applicability, and convenience.
Using chemical design synthesis, synthetic biology reconstruction, and big data model prediction, the research team from Zhejiang University discovered that the TCG codon, the least used sense codon in mammalian cells, is the “Achilles’ heel” of the entire translation system, presenting a new opportunity for advancing genetic code expansion.
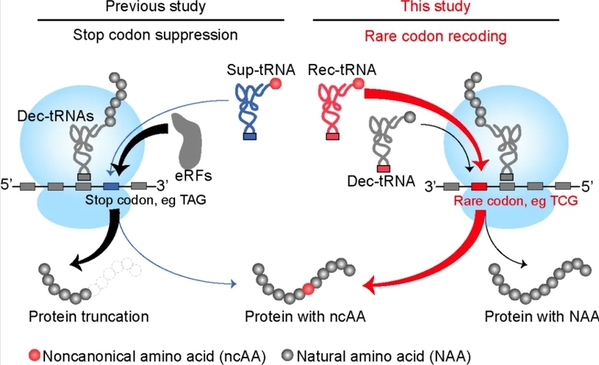
Schematic of rare codon recoding for ncAA incorporation in mammalian cells.
Creating new proteins using a “Trojan horse”
The synthesis of amino acids requires tRNA and synthetase as catalytic intermediates. The introduction of noncanonical amino acids is more than just a new breakthrough in the translation system; it necessitates leveraging the rare TCG codon to achieve this feat.
“Our team discovered that the sequence around the codon is crucial for utilizing it effectively. So, we made specific modifications to better harness it,” explained Dr. LIN Shixian.
The research team achieved this breakthrough by designing a “Trojan horse.” They loaded non-canonical amino acids onto custom-designed recoded tRNA and exploited the “Achilles’ heel” of the translation system to insert these amino acids into target proteins.
This “Trojan horse,” loaded with non-canonical amino acids, successfully infiltrated synthesis workshop of the translation system, allowing the incorporation of these amino acids into virtually any protein produced in the translation factory.
Dr. LIN Shixian added that through extensive experiments and simulation data, the team improved the recoding selectivity for non-canonical amino acids. This enabled the efficient synthesis of non-canonical amino acids without disrupting the normal synthesis of natural sequences. “We call this technology rare codon recoding, which is fundamentally different from all blank (nonsense) codon-based genetic code expansion technologies,” he noted.
Could new life forms be constructed in the future?
Both humans and mice are composed of the same 20 natural amino acids, yet they are remarkably different. Why?
“Proteins in the human body undergo literally 1,000 types of post-translational modifications, such as acetylation, phosphorylation, and ubiquitination, after they are polymerized from amino acid monomers. This makes humans much more complex than mice, fungi, bacteria, and other organisms,” explained Dr. LIN Shixian. The introduction of non-canonical amino acids can help us better understand how these protein modifications fulfill their biological functions, providing convenient research tools for understanding complex life processes.
Moreover, using non-canonical amino acid materials to create novel protein drugs could enhance drug functionality, or even design entirely new functional protein drugs. These could be used to strengthen drug-target binding, prolong drug half-life in the bloodstream, or enhance drug visibility in vivo.

Dr. LIN Shixian (second from the left) with his team in the lab
Dr. LIN Shixian envisions that in the future, based on this breakthrough, it might be possible to design cells composed of 21 or even 30 amino acids, and eventually, construct a fully functional artificial life form. “Our technology lays a solid foundation for this incredibly ‘impossible’ mission,” said Dr. LIN Shixian.
Link to the paper: https://www.science.org/doi/abs/10.1126/science.adm8143
(From ZJU NEWSROOM)

