Fluorine-containing inorganic substances are common in nature, yet natural fluorine-containing organic compounds are remarkably scarce. The vast majority of fluorine-containing organic compounds are synthesized rather than obtained in nature. Among these, compounds featuring trifluoromethyl (CF3) groups take up a substantial proportion primarily due to their ability to markedly enhance the lipophilicity and metabolic stability of compounds upon introduction. As a result, CF3 groups reign supreme in many pharmaceuticals and agrochemicals, with notable examples including Prozac for depression, Letermovir for antiviral purposes, and Lomitapide for treating high cholesterol.
The trifluoromethylation process, which involves the addition of CF3 groups to target molecules, is a highly sought-after technique in new drug development due to its role in creating high-value products. The challenge lies in introducing CF3 groups safely, efficiently, and affordably, a primary concern in the field of organic fluorine chemistry. Directly attaching CF3 groups using expensive trifluoromethylation reagents is effective but cost-prohibitive, limiting its use to research rather than large-scale production.
The research team and the reaction device they developed. (From right to left: MO Yiming, CHEN Yixin, HE Yuchen, XUAN Jun, GAO Yong)
Trifluoroacetic acid, thanks to its cost-effectiveness and operability, is hailed as an ideal source of CF3 groups in chemical synthesis, priced at a mere ~$50 per kilogram. However, its practical use is hindered by its high oxidation potential, as it necessitates harsh conditions and potent oxidants to form CF3 radicals capable of reacting directly with organic compounds. This limitation turns out to be particularly problematic for molecules containing sensitive functional groups, such as amino or aldehyde, which are widely present in pharmaceuticals and pesticides, thereby constraining the potential applications of trifluoroacetic acid. Consequently, the quest for harnessing trifluoroacetic acid’s potential under mild conditions for the production of high-value-added CF3 products remains a formidable challenge in chemical synthesis.
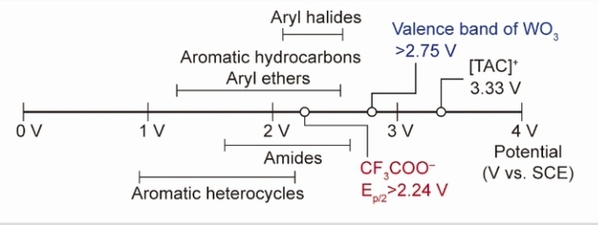
Fig. 1: Oxidation potential of CF3COO– anion, representative organic molecules, and catalysts. EOX, oxidation potential.
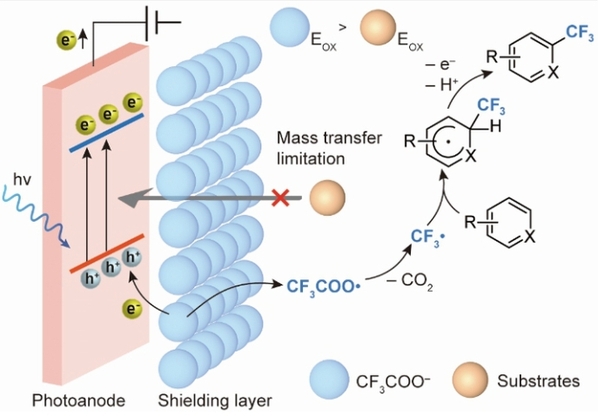
Fig. 2: Mechanism of IonShield-hPEC strategy for prioritizing electron transfer from CF3COO– anions in presence of easier-to-oxidize substrates. hv, incident photon.
In response to this challenge, the research team led by MO Yiming from the College of Chemical and Biological Engineering, Zhejiang University, developed an innovative anion-shielding heterogeneous photoelectrocatalysis strategy. This method imposes mass-transfer limitations that invert the thermodynamically determined order of electron transfer, enabling the conversion of easily oxidizable molecules into CF3 products and allowing for the synthesis of high-value-added CF3 products on a 100-gram scale. The team’s findings were published in the journal Science on May 10.
The biggest challenge in using trifluoroacetic acid directly lies in its exceedingly high oxidation potential (>2.24 V vs. SCE). When strong oxidants are applied to activate trifluoroacetic acid, other organic molecules present in the same system tend to undergo preferential oxidation.
The most straightforward solution to this problem involves preventing organic molecules from making direct contact with the oxidant. To this end, Dr. MO Yiming’s team employed photoelectrocatalysis as their method of choice. In this process, the photoanode, when illuminated, generates photogenerated holes that act as “oxidants,” while the anion layer functions as a protective barrier, stopping organic molecules from directly contacting the photoanode and thus circumventing the preferential oxidation of organic molecules. Through a series of experiments, the team proposed a selective oxidation mechanism by which “the anion layer restricts oxidation via the inhibition of mass transfer.” This led to the establishment of a new method for photoelectrocatalytic trifluoromethylation.

Fig. 3: Large-scale synthesis of DNA base analog CF3 derivative. Details of simulation, PEC-µReactor design, and scale-up procedure are available in the supplementary materials.
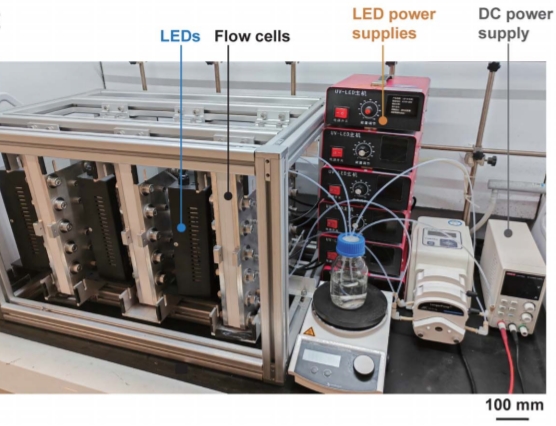
Fig. 4: Photo of the modular six-parallelized PEC-µReactor.
This method works compatibly with a variety of easily oxidizable organic molecules, and the photoelectrocatalytic system can operate stably for over 300 hours. Furthermore, using modular and scalable photoelectrochemical microreactor devices, it is possible to achieve 100-gram synthesis by using photoelectrochemical flow cells.
The study not only provides a new approach to trifluoromethylation, a synthesis technique of considerable practical value in production, but also paves the way for exploring new pathways in the study of selective electron transfer within mass transfer regulation, a critical field of inquiry in chemical engineering. This highlights the study’s significant contribution to advancing knowledge and innovation in this field.
(From ZJU NEWSROOM)

