The groundbreaking catalyst design strategy introduced by the research team, led by Prof. XIAO Feng-Shou and Prof. WANG Liang from the College of Chemical and Biological Engineering at Zhejiang University, revolves around the innovative utilization of dealuminated zeolites as carriers. This strategic implementation serves to finely regulate the dynamic evolution process of copper nanoparticles during catalytic reactions. Notably, it effectively mitigates sintering among copper particles and exerts control over already sintered copper nanoparticles for their secondary dispersion. The outcome of this approach is a significant extension of the catalyst’s lifetime. By achieving a delicate equilibrium between stability and activity, this pioneering strategy holds immense promise for applications in the development of highly durable catalysts. Their research findings were published in the journal Science on December 21, 2023.
Catalysts play a crucial role in over 90% of chemical industrial processes. Throughout these processes, they undergo dynamic structural transformations on the surface, encompassing diffusion, migration, and aggregation as an integral part of the reaction mechanism.
Evidence of this dynamic behavior is prominently demonstrated through the occurrence of sintering in many supported nano-metal catalysts after a certain period of use. Specifically, initially uniform nano-metal particles undergo a discernible shift, with disproportionately large particles emerging. The transformation from small to large particles often follows mechanisms such as “migration-aggregation” and “Ostwald ripening.” The likelihood of the latter is enhanced by chemical induction effects in the reaction atmosphere. As early as 1896, German physical chemist Wilhelm Ostwald pointed out that when solvents precipitate from supersaturated solutions, smaller particles gradually dissolve and deposit onto larger crystals or sol-gel particles.
“Catalysts typically function in high-temperature settings, often reaching temperatures of several hundred degrees Celsius. The atoms on the surface of metal particles exhibit heightened activity. Induced by chemical molecules present in the reaction atmosphere, those atoms detach and undergo rearrangement. In such instances, they tend to ‘run’ toward larger particles, driven by the lower and more stable surface energy there. Over time, large particles grow larger, while small particles become smaller and gradually dissolve,” explained Prof. WANG Liang, attributing this phenomenon to the inherent “nature” of microscopic particles.
Over the years, the “Ostwald ripening” effect has acted as an indelible “curse,” leading to irrevocable damage in catalyst performance. The number of active sites on sintered catalysts experiences a precipitous drop, resulting in a “cliff-like descent” in catalytic performance. To counter this “deterioration,” the common industrial approach involves a substantial increase in catalyst dosage or a temporary suspension of production to regenerate or replace the catalyst. Unfortunately, this corrective measure incurs high costs, similar to the routine maintenance and replacement of vehicle parts.
Don’t ‘grow up’ — this has long been the vision of scientists for supported metal catalysts. Currently, metal nano-particles are encapsulated with oxides, carbon materials, etc., to suppress metal sintering, but this process also conceals some active sites. “This strategy entails a compromise, trading some level of activity for enhanced stability,” Prof. WANG Liang said, emphasizing the pressing need for a novel approach that can achieve a balance between stability and activity.
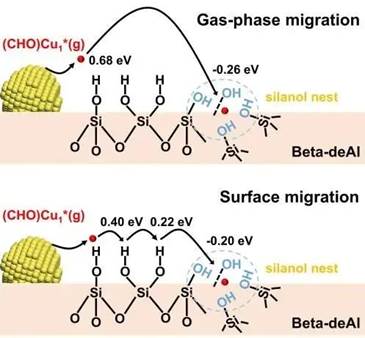
Fig. 1. Illustration showing the gas-phase and surface migration of Cu species on the catalyst surface.
The research team ingeniously engineered nano-metal particles that “don’t grow” by controlling the dynamic structural evolution pathway of metal species during the traditional sintering process. “Imagine that a celebrity is going to host a concert; to avoid overcrowding, should we encourage fans to stay at home, or should we introduce other equally or even more captivating performances in the same city to ‘induce’ people’s choices?” said Prof. XIAO Feng-Shou. “Regulating the ‘ripening’ process is, in essence, not about preventing particles from ‘running,’ but rather about redirecting the trajectory in which they ‘run’”.
In a high-temperature working environment, the introduction of a catalyst initiates “restlessness” among surface atoms. Those toms that detach from particles naturally gravitate toward the largest targets. The research team aims to reverse this situation. “We designed some ‘handles’ along the trajectory of atomic intermediate migration at the atomic level to capture these ‘running’ atoms,” explained Prof. WANG Liang. They engineered a dealuminated zeolite molecular sieve carrier for copper nano-particle catalysts, embedding numerous “silanol nests” in the carrier structure, like “pits” on a road. Through chemical reactions, these pits seize migrating intermediates, forming new nucleation sites and thus suppressing the growth of large particles.
These “pits” are not physical but chemical ones. The copper atoms that fall into these pits in the early stage undergo a chemical reaction with the hydroxyl nests of the dealuminated zeolite. This results in the establishment of a robust interaction between the intermediate migrating from the copper atoms and the carrier, making the thermodynamics of the entire process favorable. In the later stage, copper atoms on large particles continue to migrate to these new nucleation sites, ultimately reaching a dynamic equilibrium. This infusion of a new force into the original catalytic system serves to counteract the dynamic evolution process of catalyst particles. “We not only cut off the path of particle enlargement but also created new nucleation sites, ultimately reversing the process,” said Prof. WANG Liang.
Can the copper nano-particle catalysts, produced by the new strategy, truly reverse the “ripening” process and effectively extend their lifecycle? The research team tested the newly developed catalyst in the hydrogenation of dimethyl oxalate, an important reaction in the coal-to-ethylene glycol process. According to the “Ostwald ripening” effect, the overwhelming “attraction” of the initially large copper particles on the dealuminated zeolite molecular sieve would facilitate the influx of more copper atoms, resulting in continuous growth during the catalytic process.
The experimental results, however, indicate that these initially large particles gradually diminish in methanol vapor at 200°C, shrinking from an initial size of 5.6 nanometers to around 2.4 nanometers. “This observation aligns with our expectations. The dealuminated zeolite molecular sieve, rich in hydroxyl nests, successfully reversed sintering, and the copper intermediates, induced by methanol settled into these nest sites, ultimately formed new small nano-particles,” said Dr. LIU Lujie, the lead author of the study.
Interestingly, when copper powder and dealuminated zeolite molecular sieve are physically mixed and treated with methanol vapor, small copper particles are uniformly dispersed on the zeolite molecular sieve. Through mass spectrometry analysis, intermediate species that may be produced during the migration of copper atoms are observed. These observations, coupled with theoretical calculations, help elucidate the mechanism by which hydroxyl nests form stable copper nano-particles through capturing copper atoms.
The research demonstrates that that even after a long reaction time, the newly developed catalyst still maintains high efficiency. It is worth noting that this copper-based molecular sieve catalyst can also catalyze the conversion of dimethyl oxalate to ethylene glycol stably and efficiently under atmospheric pressure.
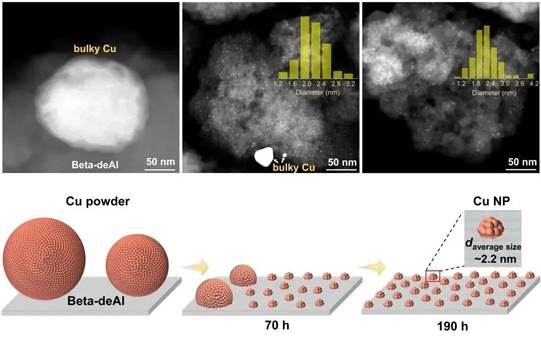
Fig. 2. Methanol-triggered structure change of the Cu particles.
“We have not only achieved the goal of reversing sintering but have also significantly improved the lifecycle of the catalyst,” said Prof. XIAO Feng-Shou. “This innovative design strategy exhibits a degree of universality, and we will explore the design and preparation of more durable catalysts in the future.”
(From ZJU NEWSROOM)

