The breakthrough achieved by chimeric antigen receptor (CAR) T cells in the treatment of hematologic malignancies undoubtedly represents the most promising approach to cancer therapy. However, CAR T cells encounter dual pressures from the immunosuppressive microenvironment and metabolic conditions after infusion into the body. The loss of memory T cells and exhaustion are crucial reasons for the failure of anti-tumor immune therapy. The mitochondria, considered the energy factory and metabolic hub of cells, undergo dynamic changes based on the biological state and nutritional environment. Mitochondrial activity and metabolic patterns are essential for the development, fate, and function of T cells. Mitochondrial metabolic abnormalities are the primary characteristics of T cell exhaustion and a key factor triggering functional impairments in T cells. However, whether targeting crucial metabolic nodes in the mitochondria can alter the fate of CAR T cells and consequently impact their anti-tumor activity remains to be fully confirmed.
Recently, Professor HUANG He from the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU), along with Professor XIAO Gang and QIAN Pengxu from Zhejiang University School of Medicine, collaborated on a research paper published in Cell Metabolism titled Mitochondrial isocitrate dehydrogenase impedes CAR T-cell function by restraining antioxidant metabolism and histone acetylation. In this study, they performed a mitochondria-related compound library screening on CAR T cells and identified isocitrate dehydrogenase 2 (IDH2) as a potential target to enhance anti-tumor function both in vitro and in vivo. Pharmacological or genetic ablation of IDH2 advanced immunotherapy, resulting in better CAR T-cell persistence and further promotion of their cytotoxicity against hematologic and solid tumors.
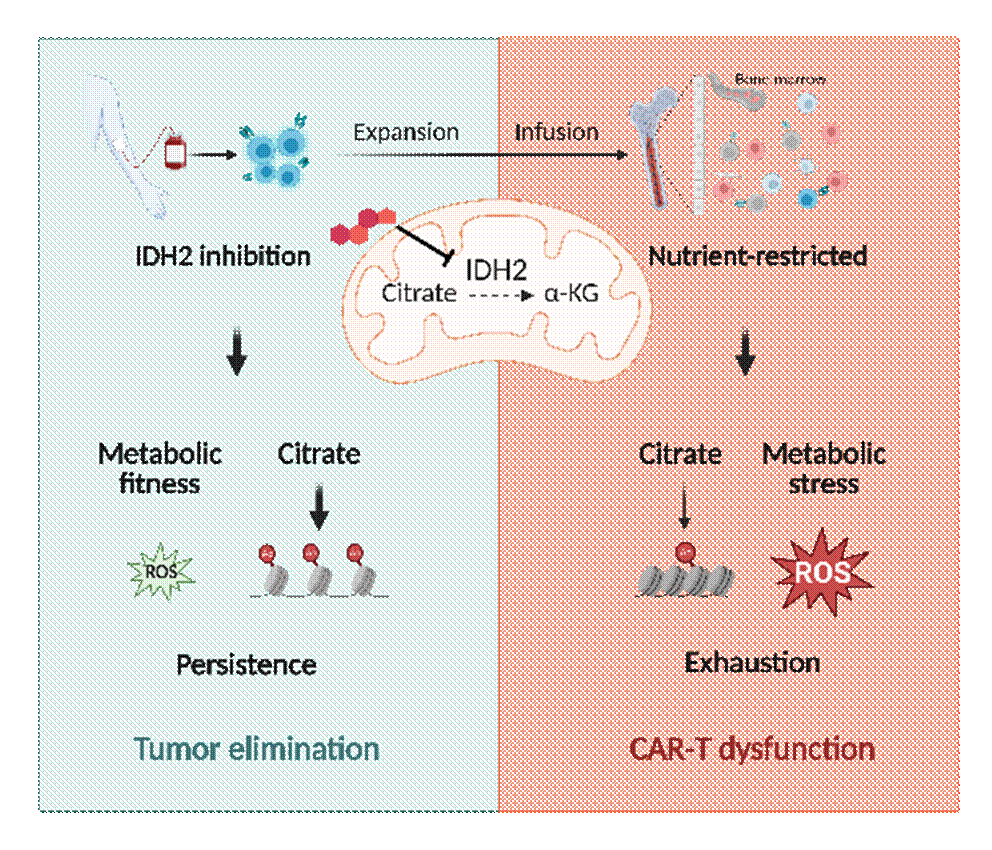
IDH2 is located in the mitochondria and utilizes NADP+ as a cofactor to catalyze the reversible conversion of isocitrate to α-ketoglutarate. Genetic knockdown or pharmacological inhibition of IDH2 can reshape the central carbon metabolism and epigenetic regulation in CAR T cells, enhancing their anti-tumor capabilities. Whether in hematologic models or solid tumor models, IDH2 inhibition can strengthen the in vivo anti-tumor abilities and persistence of CAR T cells, irrespective of the CAR architecture with different costimulatory domains and single-chain variable fragments. Multi-omics analysis and cellular metabolic activity assays confirmed that IDH2 inhibition significantly reduces glycolysis levels, redirecting glucose utilization in CAR T cells from glycolysis to the pentose phosphate pathway (PPP). This shift leads to higher proportions of NADPH and GSH, reducing ROS levels and effectively inhibiting CAR T cell exhaustion. The detection of citrate and acetyl-CoA levels in mitochondrial and cytoplasmic fractions confirmed that IDH2 inhibition results in the accumulation of citrate in the cytoplasm, directly increasing acetyl-CoA and histone acetylation levels by blocking the oxidative decarboxylation reaction. Integrative analysis of CUT&Tag-seq and RNA-seq revealed that increased epigenetic modifications mediate transcriptional reprogramming, promote the formation of memory T cells and enhance the long-term survival of CAR T cells. The study also discovered that CAR T cells undergo terminal differentiation and functional impairment when transitioning from a high glucose environment in vitro to glucose deprivation in vivo, and IDH2 inhibition promotes the metabolic robustness and tumor-killing function of CAR T cells under a nutrient-restricted condition.
Graphical abstract
Currently, it is widely acknowledged that metabolic patterns are playmakers of the fate and longevity of T cells. The challenge lies in precisely targeting metabolic regulation nodes to enhance the anti-tumor effects of CAR T cells while avoiding toxic side effects. This study utilized the FDA-approved leukemia clinical drug Enasidenib to enhance the persistent anti-tumor effector of CAR T cells, and dissected the role of mitochondrial metabolism in determining the fate of CAR T cells. This research provides a safe intervention strategy to enhance the metabolic adaptability and therapeutic effects of CAR T cells. Professor HUANG He's team has been engaged in fundamental and clinical research on hematopoietic stem cells and cell immunotherapy. In recent years, the research group has conducted innovative studies in the development of enhanced immune cell therapy and the construction of novel immune cell therapies from stem cells. Simultaneously, they have explored the application of immunotherapy in refractory and recurrent hematologic malignancies, as well as major autoimmune diseases. Notably, their recent achievements have been published in journals such as Nature, The Lancet Haematology, Cell Research, Blood, and Nature Communications. Additionally, the team has also initiated and participated in several internationally leading clinical studies.
More information: Postdoctoral researcher SI Xiaohui from FAHZU, along with doctoral students SHAO Mi, TENG Xinyi, and HUANG Yue, are the co-first authors of this paper. MENG Ye and WU Longyuan have made significant contributions to this research. Professor HUANG He, XIAO Gang, and QIAN Pengxu are the corresponding authors of this paper. The study received support from the core facilities of Zhejiang University School of Medicine and the Liangzhu Laboratory. Guidance and assistance were also provided by Professor Huang Peng from Sun Yat-sen University, Professor Pei Shanshan and Professor Guo Hongshan from Zhejiang University.
Source: The First Affiliated Hospital, Zhejiang University School of Medicine
(From ZJU NEWSROOM)

