Vaccines are essential for protecting human from infectious disease. They contain antigens derived from pathogens or synthetically created subunits that mimic the pathogen's components. Vaccines are generally classified as live attenuated vaccines that are engineered by reducing the replication of pathogenic strains, and non-live vaccines contain only pathogen components or killed whole organisms. However, there are concerns about reduced immunogenicity, safety, and time-consuming manufacturing processes associated with current virus vaccines developing strategy, which hampers their widespread use. There is a notable impetus to develop next-generation vaccine technology that rapidly converts a pathogenic virus into vaccines with high safety and effectiveness.
Recently, Prof. TANG Ruikang’s group at the Zhejiang University Department of Chemistry, proposed a ready-to-use vaccine development strategy by directly entrapping viruses in a cationic chitosan hydrogel. This technology enables a virus-to-vaccine conversion via in situ prisoning and eliminating viruses while enlarging their specific immune responses. Their findings have been published in the journal Nature Biomedical Engineering.
TANG Ruikang’s group abandoned traditional ideas of modifying viruses themselves and sought a new approach by rationally designing a virus-entrapped microenvironment. They identified chitosan oligomer hydrogel, a commonly used medical polymer extracted from shrimp and crab shells, as an ideal virus trapping materials. They prepared a chitosan-based hydrogel which contains many trap-like pores, and when live viruses are loaded inside, their negative charge attracts the positive charged chitosan scaffold in the pores, trapping the viruses.
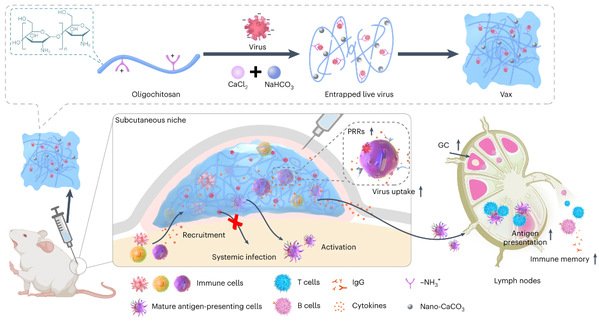
Fig. 1: Schematic of the virus-entrapping hydrogel (Vax).
Additionally, the unique water channel in the hydrogel allows immune cells to enter smoothly. The team also loaded calcium carbonate nanoparticles (nano-CaCO3) to attract more immune cells, which help to engulf viruses by activating the pathogen recognition receptors of these immune cells. The virus-trapped hydrogel is named Vax.
Vax not only trapped viruses but also recruited immune cells to kill viruses. Immune cells can engulf viruses while processing antigen information and then present it to lymph nodes, enabling an adaptive immune response to produce antibodies throughout the body. Dr. WANG Xiaoyu, one of the corresponding authors from Qiushi Academy for Advanced Studies at Zhejiang University, emphasized the effectiveness of using real viruses to mount the immune system instead of fake viruses used in past simulations. “It is agreed that gaining immunity in a real battle is more effective.”
Safety and effectiveness are fundamental benchmarks for assessing vaccine quality. TANG's team, after conducting basic experiments, collaborated with the Beijing Institute of Biotechnology to conduct animal experiments on the Zika virus (ZIKV). ZIKV is mainly transmitted through mosquito bites, and there is currently no available vaccine. A single-dose vaccination prepared by loading live pathogenic ZIKV into the Vax without prior treatment can evoke effective anti-ZIKV immunity and protect mice against lethal infection with a survival rate of 100% .

Fig. 2: ZIKV-loaded Vax ensured a protective immune response against ZIKV infection. (A) Survival curves of immunized IFNAR1−/− mice after lethal challenge with ZIKV. (B) Mouse weight after challenge.
However, there were still doubts whether this strategy is 100% safe. Safety was always a top priority for the team, and they spent five years optimizing relevant parameters to ensure that the hydrogel, the immune factory, only exported antigenic products to the body meanwhile the virus was firmly circumscribed to the hydrogel. Experiments showed that no viral component was detected in major organs of the mice, including the liver, kidney, brain, and heart 52 days after injection of the wild Zika virus-loaded hydrogel.
“The usual development cycle for a vaccine is 8 to 10 years. The highlight of this study is that there is no need to process the virus to make it less virulent and less infectious, as is typically required to develop traditional vaccines. Instead, directly loading a live virus strain into a specific material can quickly prepare a safe and efficient vaccine. “TANG explained. This breakthrough technology has the potential to rapidly and safely develop vaccines, representing a promising material-based strategy to enable a virus-to-vaccine conversion.

Prof. TANG Ruikang (the second from the left) and Dr. WANG Xiaoyu (the second from the right) were discussing with their team in the lab.
TANG 's team is aiming to change lives with materials, and their proof-of-concept study may open up a new avenue for vaccine development. TANG Ruikang hopes that more scientists would follow in their footsteps and promote this concept into reality as soon as possible.
(From ZJU NEWSROOM)

