Liver cancer is the fourth leading cause of cancer-related death globally. It is indeed the second most lethal tumor. Hepatocellular carcinoma (HCC) is the major type among them. Recently, it was noticed that metabolic dysregulation contributed a lot to the progression of HCC, whereas the roles of metabolic dysregulation in HCC treatment has not been fully revealed. Sorafenib has been a standard targeted therapy for advanced HCC, where metabolic aspect especially ROS is deeply involved. Sorafenib and other emerging novel TKIs have been clinically effective for controlling HCC progression; however, drug resistance curtails their efficacy. Understanding the molecular mechanisms of sorafenib resistance may provide novel therapeutic targets and more insights for combination therapies for the management of HCC. The role of metabolic reprogramming and redox homeostasis in the process of sorafenib resistance has remained unknown.
Recently the research team led by Prof. CAI Xiujun from Sir Run-Run Shaw Hospital of the Zhejiang University School of Medicine has found something important and interesting in this area. It has been recently published in Signal Transduction and Targeted Therapy as a cover story, entitled “UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1β in hepatocellular carcinoma”. They explored the role of UBQLN1 in sorafenib resistance in perspective of mitochondrial biogenesis and ROS homeostasis.
Sorafenib was reported to target ETC complexes that directly induce ROS generation in HCC cells. Therefore, to maintain sorafenib resistance and further facilitate tumor progression, cancer cells develop strategies to overcome excessive ROS production to obtain resistance to oxidative stress-induced cell death. In this study, Xu et al. found that reactive oxygen species (ROS) levels decreased in sorafenib-resistant cells compared with parental cells, indicating that the ROS and redox homeostasis is crucial for sorafenib resistance development.
Mitochondria are the first site of ROS production and redox homeostasis, and hypoxia increases ROS levels mainly through affecting the mitochondria. Thus, researchers turned to mitochondria for further investigations. They found less but healthier mitochondria in sorafenib-resistant cells, and more but damaged mitochondria in parental cells. Was it caused by augmented mitophagy? Researchers were intrigued to investigate autophagic flux. However, autophagic flux was reduced in sorafenib-resistant cells, which could not explain the short of mitochondria. Was it caused by reduced mitochondrial biogenesis? Researchers evaluated the levels of two key proteins, PGC1α and PGC1β regulating mitochondrial biogenesis, and found that PGC1β was significantly reduced in sorafenib-resistant cells. Over-expressing PGC1β could accelerate ROS production in sorafenib-resistant cells, suggesting that reduced mitochondrial biogenesis is likely to lead to reduced ROS production, thus a reduced stress for cells and a better adaption to sorafenib treatment.
Increased ubiquitin-mediated proteolysis of PGC1β was found in sorafenib-resistant cells, which contributed to decreased mitochondrial biogenesis and ROS generation. Xu et al. further characterized a role for UBQLN1 in mediating PGC1β ubiquitination and degradation. UBQLN1 has been widely studied in neurodegenerative diseases and was found to be deregulated in various disorders ranging from Alzheimer’s disease to cancer. As a UBL-UBA protein, UBQLN1 is assumed to bind to poly-ubiquitin chains of substrate and shuttle it to the proteasome. Some studies demonstrate that UBQLN1 facilitates the proteolysis of it bound substrates, while other studies also revealed that UBQLN1 stabilizes proteins that it binds, indicating that UBQLN1 either facilitates or retards the degradation of its bound substrates. In this study, Xu et al. found that UBQLN1 serves as a degradation promoter through its chaperone function to facilitate the interaction between PGC1β and proteasome in a ubiquitination-independent manner. The target-dependent functions of UBQLN1 are very important for its potential value of engineered targeted proteolysis, similar to PROTAC, which needs further investigations.
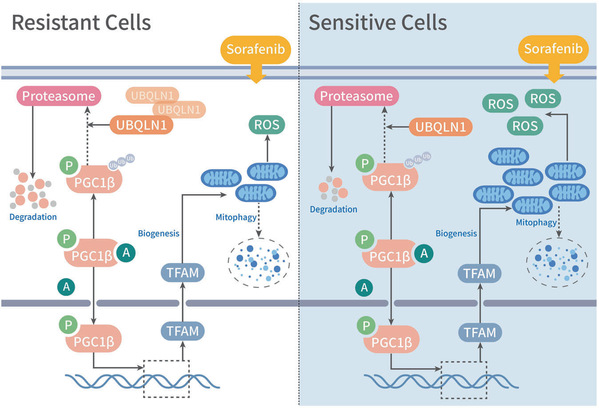
“This study reveals that UBQLN1-PGC1β-mediated mitochondrial biogenesis and ROS homeostasis play critical roles in sorafenib resistance in HCC, providing novel targets for combination therapies and pharmaceutical development,” said CAI Xiujun.
(Source: Sir Run-Run Shaw Hospital, Zhejiang University School of Medicine)

