If you were to divide 100 students into two groups, what would you do? Would you split them up at random or would you divide them in accordance with certain traits, say, boys or girls? Apparently, the former is far easier.
As a matter of fact, an act like this is staged in our living organism at every moment. This is technically termed as cell division, the process by which a parent cell divides into two or more daughter cells. Conventional wisdom has it that cell constituents are equally distributed between the two daughter cells during cell division. However, this idea has been challenged by the likelihood that chromosomes segregate non-randomly during mitosis. However, the mechanism underlying nonrandom separation of sister chromatids has remained elusive.
Recently, the research team headed by Prof. YING Songmin and Prof. SHEN Huahao from the Zhejiang University School of Medicine observed non-random chromatid segregation during mitosis: the DNA in one sister cell is intact while that in the other sister cell is severely damaged with a salient DNA damage response (DDR). Researchers discovered that the ATR/CHK1 signaling pathway plays an essential role in mediating non-random DNA segregation (NDS), suggesting that the living organism saves its gene in a very smart manner. Their research findings are published open access in the April 30 issue of the journal of Molecular Cell.
About half a century ago, scientists found that when cells are not randomly distributed, the mother cell will be divided into two different daughter cells, a “new” cell and an “old” cell.
In 2013, YING returned to China and became a faculty member in the ZJU community. Once he led a cohort of undergraduates to perform an innovative experimentand observed a pair of “twin” cells which resembled each other outside but differed from each other inside by happenstance. This was a very rare phenomenon of asymmetric distribution in cell division.
By adopting immunofluorescence staining, YING discovered that there existed an enigma in this pair of “twin” cells. The inherited DNA in the “old” cell was intact while the acquired DNA in the “new” cell was subjected to much damage. “On the surface, they were distinct from each other in their newness, but in essence, it is due to the distribution of damaged and non-damaged DNAs,” YING remarked.
It turns out that cell proliferation and cell division are fraught with various challenges. For example, DNA damage is likely to occur during these processes. In this case, cells may activate a network of DDR proteins in an attempt to resolve any DNA damage, but sometimes damaged chromosomes fail to recover completely prior to cell division. If random distribution occurred, damaged chromosomes would be passed on to both daughter cells simultaneously, which would proliferate exponentially in the succeeding cell division, thereby causing immeasurable outcomes.
At this time, “isolation” is also a kind of love. The mother cell allocates its DNA in a very ingenious way during cell division. The non-damaged DNA is transmitted to one of the daughter cells so that it can preserve its proliferation capability while the damaged DNA is isolated and passed onto the other daughter cell which is prone to cell-cycle arrest or cell death.
“It is due to this ‘isolation’ that healthy daughter cells can develop and proliferate whereas damaged ones will be eliminated,” YING said, “Isolating damaged cells may play a key role in ensuring the long-term survival of living cells during the development of stem cells. This involves a very basic life phenomenon regarding how cells transmit intact genetic information into their daughter cells in the process of evolution.”
In humans, each cell normally contains 23 pairs of chromosomes. The distributional probability of each pair is 50-50. Without an efficient regulatory mechanism, the probability of non-random DNA segregation is 1 in 223. This probability would be very infinitesimal, which could explain why asymmetric distribution is scarcely observable and rarely studied.
Among 100 cells, less than 10 cells are in the process of cell division, and most of the cells are randomly distributed. At rough estimates, one would have to observe tens of thousands of cells before finding a cell that is not randomly distributed. “It is a herculean task to look into the confocal microscope for a full day.”
Another prerequisite for non-random segregation is that damaged chromosomes have not yet been repaired before cell division. So under what conditions does DNA damage occur? During the DNA replication process, there exist various situations where, like Tetris, not all places are seamless and there may be a mismatched tile or an empty space. In addition, the telomere portion of the DNA is far from easily replicated. Once poorly replicated, the cell will be damaged. This replication disorder is called replication stress. Some of the minor injuries will accumulate after non-random segregation, thereby resulting in DNA injuries all over the affected cell.
DNA damage can also be caused by such internal factors as activation of the proto-oncogene and such external factors as chemical agents that affect DNA replication. When these injuries are not perfectly repaired, asymmetric distribution may well be triggered.
In normal cells, non-random segregation is the last line of defense for the proliferation of life, ensuring the transmission of intact chromosome information from generation to generation. In contrast, in tumor cells, non-random segregation may favor tumor growth. Although most of the cells with ‘‘damaged’’ DNA would be expected not to survive, some might inadvertently acquire traits that provide a selective advantage, permitting the development of more aggressive or invasive tumors.Understanding the story behind the occurrence of non-random segregation can provide an insightful exploration of the causes of rapid tumor proliferation and drug resistance, thus opening upa potential targeting option for precision tumor therapy.
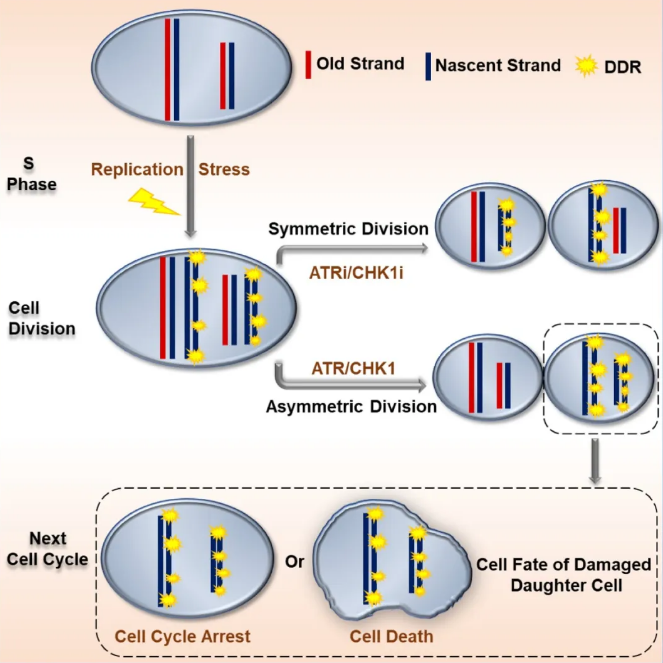
During this research, YING et al. reveal that the ATR/CHK1 signaling pathway plays anessential role in mediating NDS. This biased segregation process contributes to cell-cycle arrest andcell death in damaged daughter cells inheriting newly replicated DNA. These data therefore identify a key rolefor NDS in the maintenance of genomic integrity within cancer cell populations undergoing replication stress due to oncogene activation.
“This research offers a novel perspective,” YING said, “The living organism is remarkably smart. Once a cell is damaged, it will repair itself spontaneously. If repair is not successful, there will be the last line of defense—isolating the damaged portion by non-random DNA segregation, thus ensuring the maximum benefit of the whole community.”

Prof. YING (third from right) with his students
(From ZJU NEWSROOM)

