As a clean energy source, hydrogen is characterized as pollution-free, high in combustion value and abundant. Water can be split into hydrogen and oxygen via electrolysis. The electric/ photoelectrocatalytic oxygen evolution reaction (OER) process is a complex reaction process involving four-electron transfer, marked by slow-reaction kinetics and high overpotentials, thereby inhibiting the overall energy conversion efficiency.
Researchers have developed Iridium-based materials as state-of-the-art electrocatalysts for OER, but their staggering cost poses a significant barrier to their widespread applications. Motivated by this challenge, appreciable efforts have been devoted to developing inexpensive non-precious metal OER electrocatalysts. Ideal OER electrocatalysts for PEC water splitting require not only small overpotentials and low costs for OER but also stable electrical contacts with the photoelectrode.
Recently, Dr. HOU Yang, a principal investigator of the College of Chemical and Biological Engineering, has developed a nanocarbon OER electrocatalyst composed of atomically disperse S|NiNx species embedded in porous carbon (PC) nanosheets (denoted S|NiNx−PC), which are synthesized by the pyrolysis of ternary dicyandiamide–thiophene–nickel salt nanocomposites grown on electrochemically exfoliated graphene (EG) foil. This finding is published online in the journal of Nature Communications.
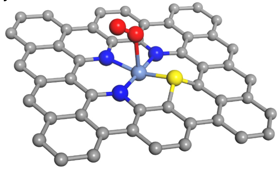
Schematic structural model for S|NiNx−PC. The steel blue, blue, yellow, gray, and red spheres represent Ni, N, S, C, and O atoms, respectively
The atomically dispersed nickel–nitrogen–sulfur species anchored on porous carbon nanosheets exhibits remarkable activity and salient durability for OER with a low overpotential of 1.51 V at 10 mA cm−2 and a small Tafel slope of 45 mV dec−1 in alkaline media. Such electrocatalyst represents the best among all reported transition metal- and/or heteroatom-doped carbon electrocatalysts and is even superior to benchmark Ir/C. Theoretical and experimental results demonstrate that the well-dispersed molecular S|NiNx species act as active sites for catalyzing OER. The atomic structure of S|NiNx centers in the carbon matrix is clearly disclosed by aberration-corrected scanning transmission electron microscopy and synchrotron radiation X-ray absorption spectroscopy together with computational simulations. An integrated photoanode of nanocarbon on a Fe2O3 nanosheet array enables highly active solar-driven oxygen production.

Dr. HOU (left)with his team
Cost-wise, it is 80% lower than commercial Iridium catalyst, said HOU. “The stability is also much improved, which show a great promise to produce hydrogen through water-splitting in an industry scale.”
“Furthermore, this catalyst design may illuminate the development of novel carbon materials with atomically disperse active molecular entities for diverse PEC applications, including CO2 reduction, oxygen reduction, and nitrogen fixation,” added HOU.
From ZJU NEWSROOM

